It's really not as simple as far too many of today's new found 'armchair experts' like to think!
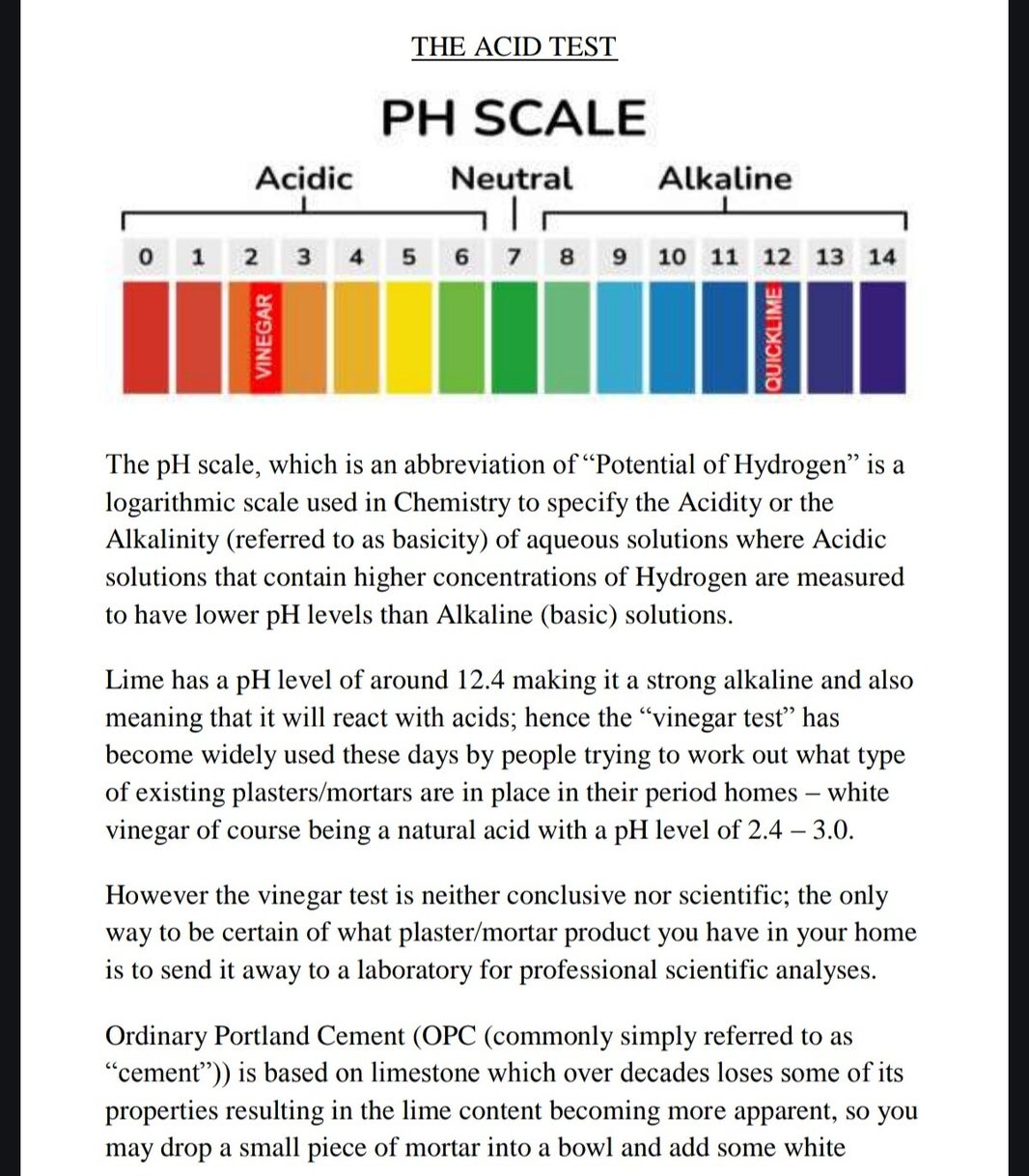

We"veall heard about uthe vinegarvtest for determining lime or cement: you take ja small piece of the plaster, render, Mortar or whatever else you are unsure of and you put it in a bowl or Even on a plate and you simply add vinegar to it. The thinking behind this is simply that lime is an Alkaline and Vinegar is an Acid, so the two will react with one another and this is seen by 'fizzing' of the Vinegar (acid) but there's one or two things that simply do not make this true., it's a thinking, but it's not science.
First we need to understand what OPC means and what cement is made of
OPC is the common abbreviation used in the construction industry for Ordinary Portland Cement - Approximately 40% to 60% of 'cement' is limestone which has been heated in a kiln to drive out moisture and CO², and then crushed to a powder, and this is what gives cement the bulk of it's calcium content.
- About 20-25% of cement includes clay, silicates, alumina, and/or iron oxide.
- Some manufacturing plants will also mix in ground shells, to further increse the calcium content.
- Silicate accounts for around 5-10% of the overall cement mix, increasing both it's strength and durability.
(Hopefully you will have noticed that there's quite a large Elephant already in in the room!, in fact there's two!)
Limestone is, as the name says, Lime, so how do you know that the vinegar
'fizz' is really because it's pure lime or an older, deteriorating cement?
Another issue to be aware of is that cement and lime have been a regular mix since the late 1800's, frequently used in early cavity walls to create a pH neutral mix (calcium carbonate, 7 on the pH scale) which wouldn't corrode the early steel cavity wall ties, (there is of course acid erosion, but Alkaline erosion is also very real, so in these instances which one will your 'magic' vinegar test choose?
But, do remember that mould hates vinegar!